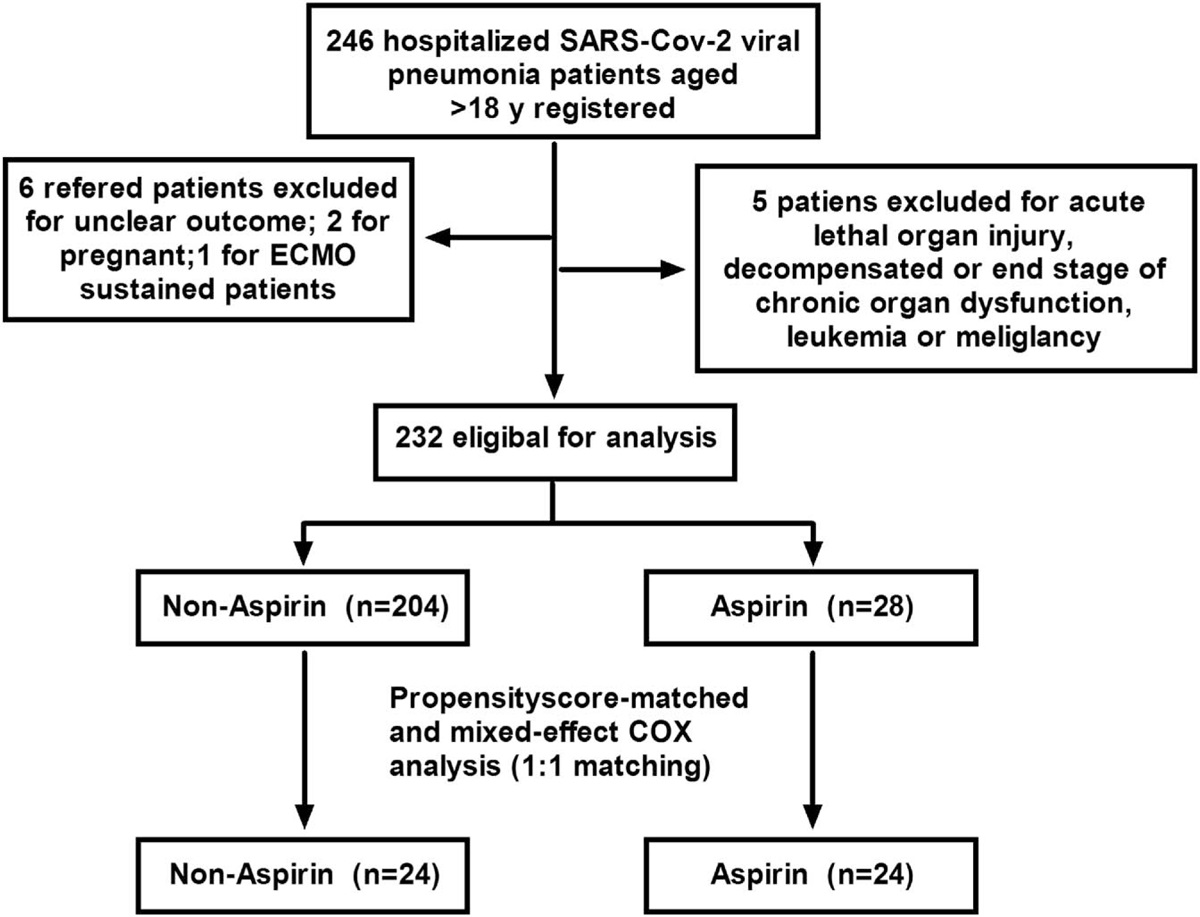
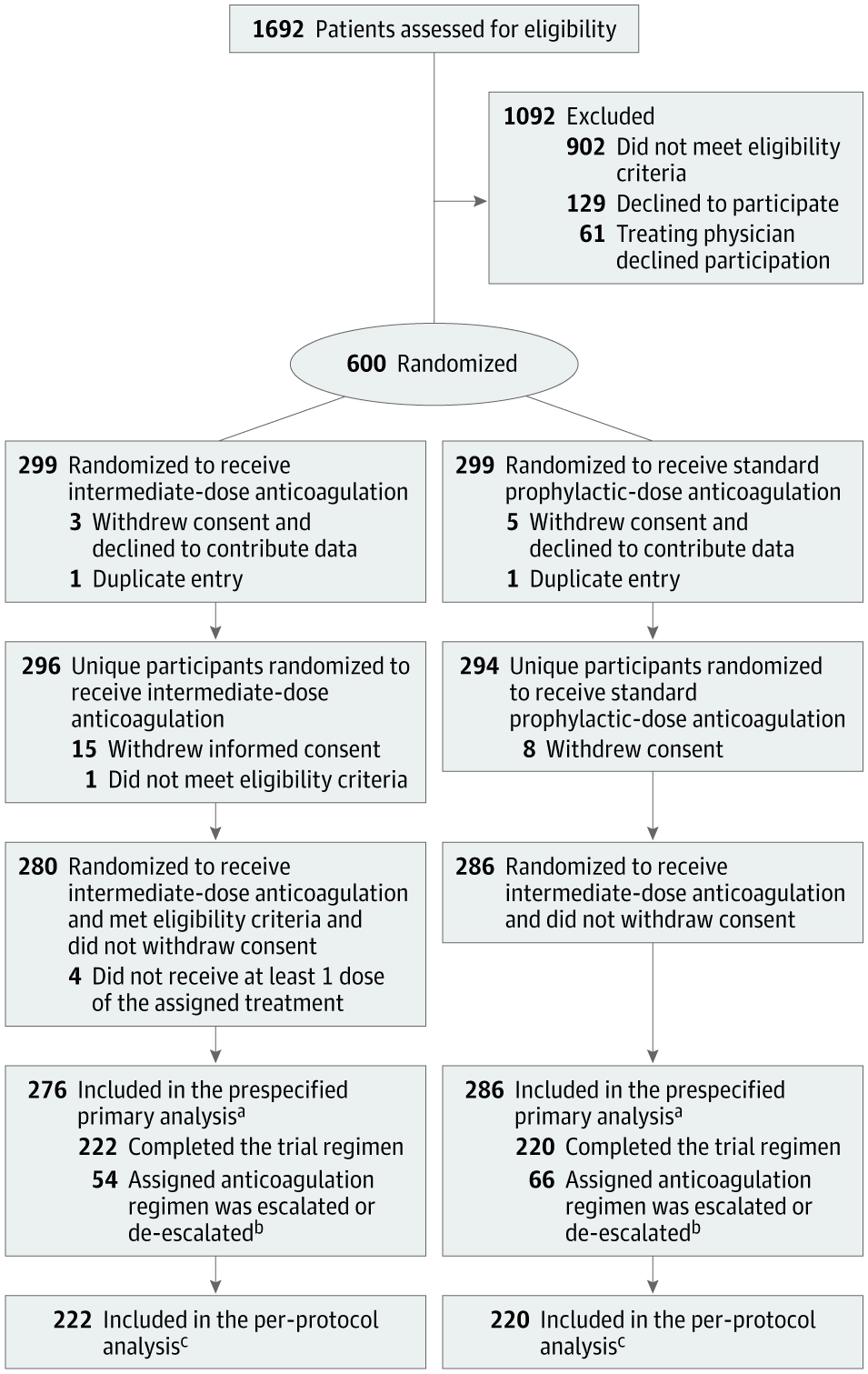
New British (Kent) Strain could be up to 33% more lethal
Newer researches and preliminary findings from the London School of Hygiene and Tropical Medicine (LSHTM) have also identified that the SARS-COV-2 variant may also increase the risk of dying from the disease by a whopping 33%.
 timesofindia.indiatimes.com
timesofindia.indiatimes.com
It is 50-74% more infective
Newer researches and preliminary findings from the London School of Hygiene and Tropical Medicine (LSHTM) have also identified that the SARS-COV-2 variant may also increase the risk of dying from the disease by a whopping 33%.

What do the studies tell us? | The Times of India
To ascertain the increased mortality risk associated with the dominant strain, London scientists identified over 8,50,000 people who tested positive for the virus between November 2020-January 2021.With the presence of the new variant, it has also been seen that the mortality risk for the virus...
It is 50-74% more infective