JackGabriel
Oakley Beginner
- 1
- 51
Hi I'm a psychiatrist and I want to help people fighting depression and I have a medicine named Rivotril 2mg that helps a person to be better again.Most COVID-19 is Spread by 18-24 yo (USA)
57 percent of those new cases occurred in people 18 to 24 yo.
“It has been reported that mitigation behaviors, such as social distancing, wearing masks, and avoiding crowded spaces, is lowest among people between the ages of 18 and 29,” he told Healthline.
“They’re more likely to be asymptomatic and can easily unknowingly transmit the virus to others,” Russell said. “Many young adults also know that their risk of lethal infection or developing long-term health problems as a result of the virus is very low, which decreases their anxiety about getting sick and lends them less reason to adhere to COVID-19 recommended practices.”

COVID-19 Trends Among Persons Aged 0–24 ...
Coronavirus disease 2019 (COVID-19) case and electronic laboratory data reported to CDC were analyzed to describe demographic characteristics, underlying health conditions ...www.cdc.gov
Recent hospitalizations among children for COVID-19 were nearly 9 times higher than last spring.
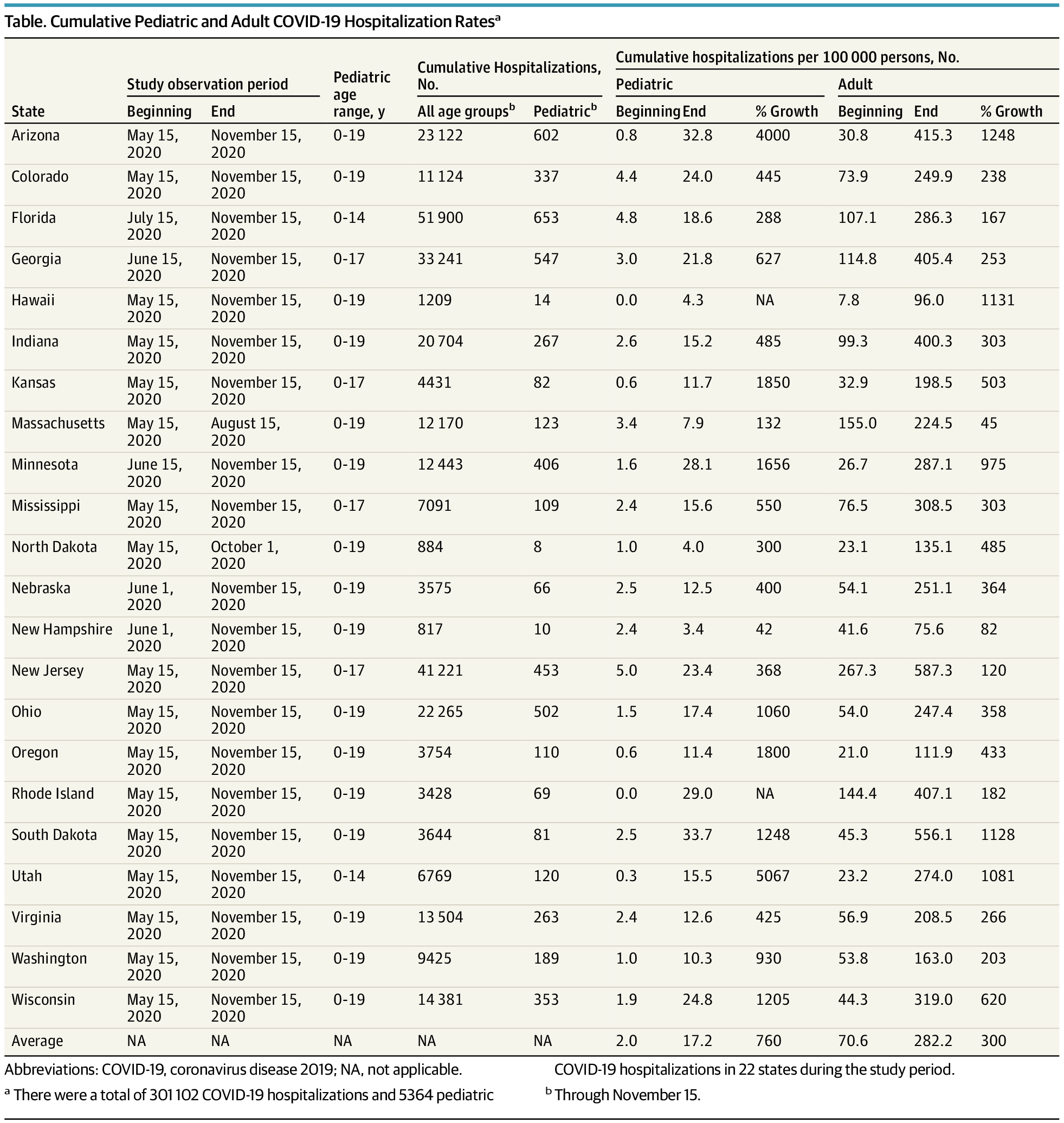
Trends in Pediatric Hospitalizations for Coronavirus Disease 2019
This study examines coronavirus disease 2019 hospitalization trends for pediatric patients in 22 states.jamanetwork.com