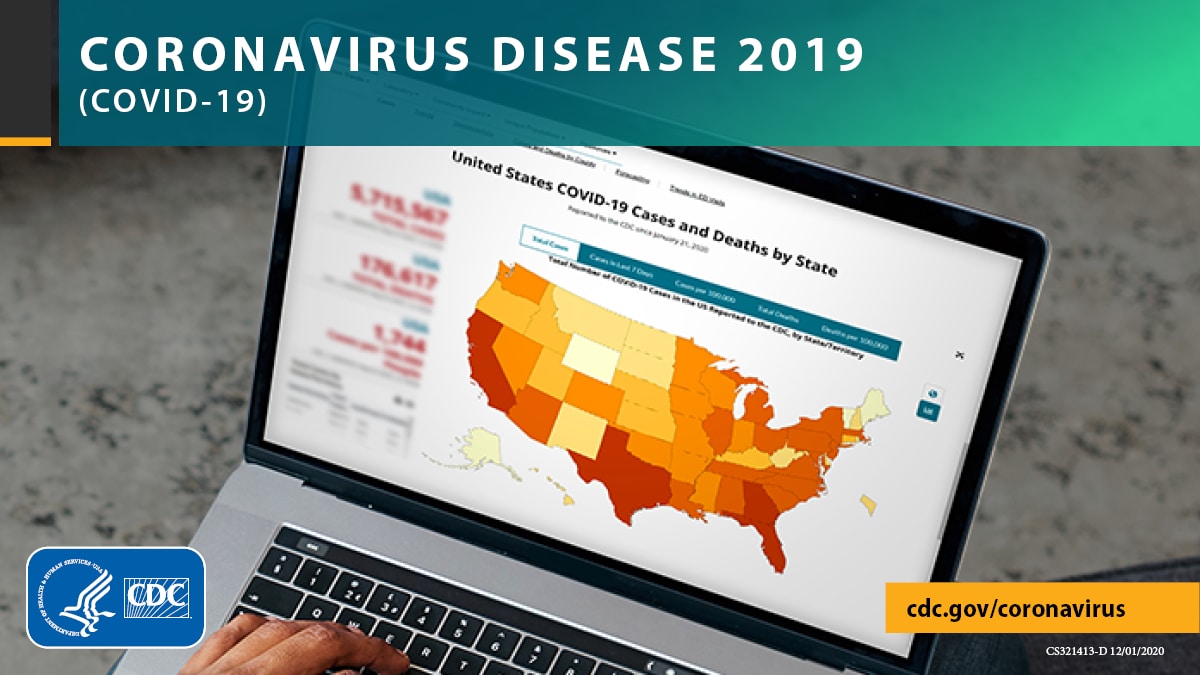
Moderna Vaccine - Protective Against the Newer Strains
The two-dose regimen of the Moderna COVID-19 Vaccine at the 100 µg dose is expected to be protective against emerging strains detected to date.
 investors.modernatx.com
investors.modernatx.com
Moderna announced today its mRNA-1273 COVID-19 vaccine does produce neutralizing titers against the B.1.1.7 (UK variant) and B.1.351 (South Africa variant). However, the company said there is a six-fold reduction in neutralizing titers in the latter variant.
 www.contagionlive.com
www.contagionlive.com
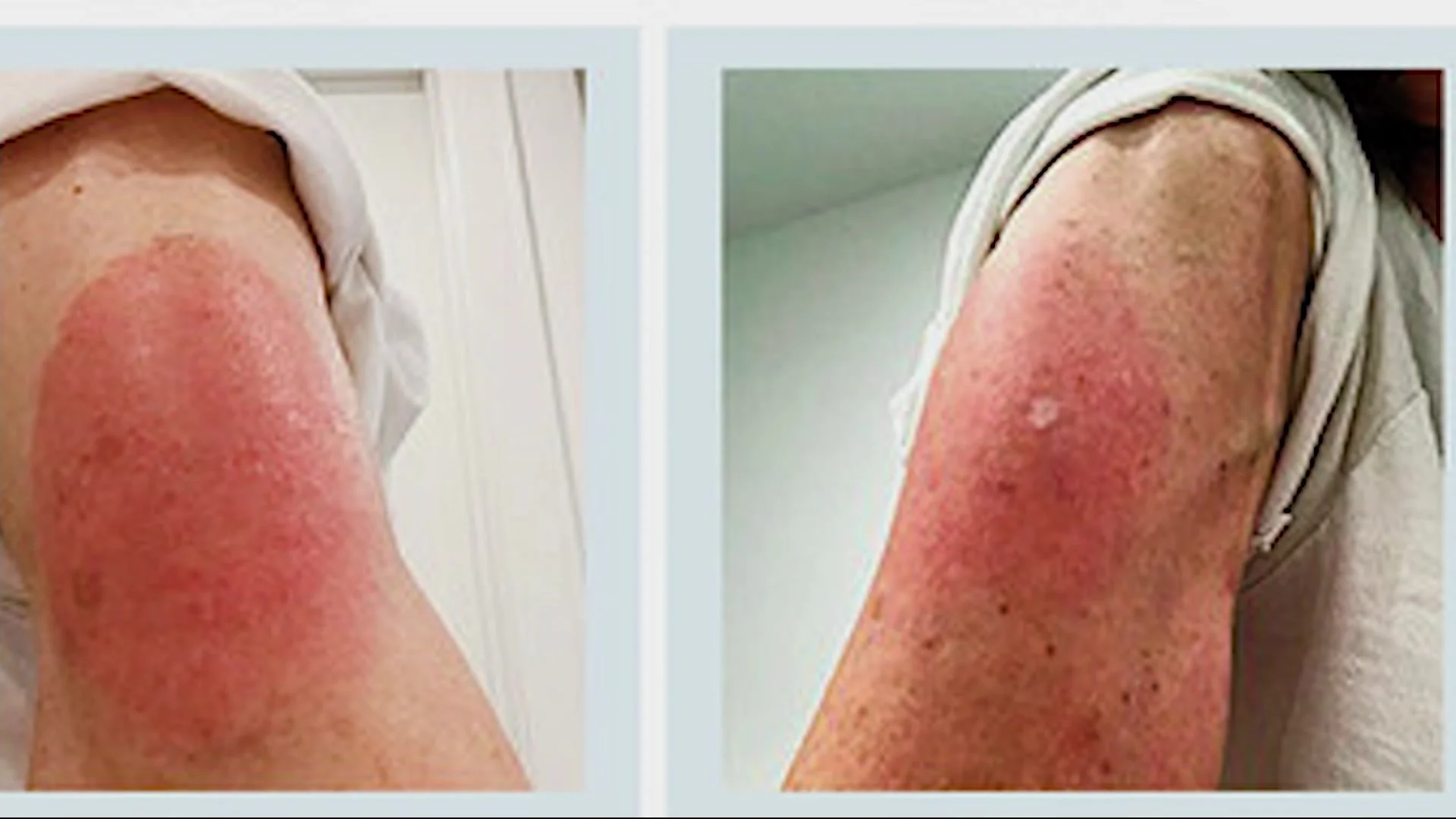
Out of 100 people who get a Moderna shot, less than 1% develop a skin reaction of this type. After 8-10 days post vaccination. Treatment is supportive therapy only.
Antibodies are still present 6 months after 2nd dose
Good for 6 months

 www.reuters.com
www.reuters.com
The vaccine proved 96% effective against Covid-19 hospitalization and 87% effective in preventing infection in a study of more than 700,000 individuals—half fully vaccinated, half unvaccinated—at Kaiser Permanente Southern California, the company said, noting the delta variant made up 47% of the cases among vaccinated individuals.
The company also highlighted a recent study by the CDC of more than 33,000 medical encounters in nine states which found the Moderna vaccine to be more effective than others, with 95% efficacy against hospitalization and 92% efficacy against urgent care and emergency visits from Covid-19 (the delta variant was the dominant variant during this time).
Looking at more than 26,000 people who received at least one dose of the vaccine, Moderna found 162 breakthrough cases among the 16,747 vaccinated between July and August 2020 compared to 88 among the 11,431 inoculated between December 2020 and March 2021, meaning the rate of breakthrough infections was about 36% lower among those vaccinated more recently (when adjusted for the study’s timing).

 www.forbes.com
www.forbes.com
The two-dose regimen of the Moderna COVID-19 Vaccine at the 100 µg dose is expected to be protective against emerging strains detected to date.
Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emerging Variants First Identified in the U.K. and the Republic of South Africa | Moderna, Inc.
Out of an abundance of caution, Moderna launches clinical program to boost immunity to emerging variants Manuscript posted to preprint server; company to host conference call once manuscript is available CAMBRIDGE, Mass. --(BUSINESS WIRE)--Jan. 25, 2021-- Moderna Inc .
 investors.modernatx.com
investors.modernatx.com Moderna announced today its mRNA-1273 COVID-19 vaccine does produce neutralizing titers against the B.1.1.7 (UK variant) and B.1.351 (South Africa variant). However, the company said there is a six-fold reduction in neutralizing titers in the latter variant.

Moderna Vaccine Offers Protection Against New COVID-19 Variants
However, there is a six-fold reduction in neutralizing titers in the B.1.351 variant from South Africa, so the company is exploring strategies aimed against new variants.
Out of 100 people who get a Moderna shot, less than 1% develop a skin reaction of this type. After 8-10 days post vaccination. Treatment is supportive therapy only.

Medical experts: Rash could develop at injection site of Moderna COVID-19 vaccine
Medical experts are making the public aware of a skin irritation that may develop in some people days after receiving a Moderna coronavirus vaccine.
bronx.news12.com
Antibodies are still present 6 months after 2nd dose
Good for 6 months

Moderna says protection from its COVID-19 vaccine still strong six months on
Moderna Inc said on Tuesday that its COVID-19 vaccine still showed strong protection against the illness six months after people received their second shot, with efficacy of more than 90 percent against all cases of COVID-19 and more than 95 percent against severe COVID-19.
The vaccine proved 96% effective against Covid-19 hospitalization and 87% effective in preventing infection in a study of more than 700,000 individuals—half fully vaccinated, half unvaccinated—at Kaiser Permanente Southern California, the company said, noting the delta variant made up 47% of the cases among vaccinated individuals.
The company also highlighted a recent study by the CDC of more than 33,000 medical encounters in nine states which found the Moderna vaccine to be more effective than others, with 95% efficacy against hospitalization and 92% efficacy against urgent care and emergency visits from Covid-19 (the delta variant was the dominant variant during this time).
Looking at more than 26,000 people who received at least one dose of the vaccine, Moderna found 162 breakthrough cases among the 16,747 vaccinated between July and August 2020 compared to 88 among the 11,431 inoculated between December 2020 and March 2021, meaning the rate of breakthrough infections was about 36% lower among those vaccinated more recently (when adjusted for the study’s timing).

Moderna Vaccine Highly Effective Against Delta—But Protection Against Infection May Wane Over Time, New Data Suggests
The company said it believes booster shots are necessary “to maintain high levels of protection” as it saw 36% more “breakthrough infections” in people who got the vaccine last year.
 www.forbes.com
www.forbes.com Last edited: