Vaccinated vs Unvaccinated
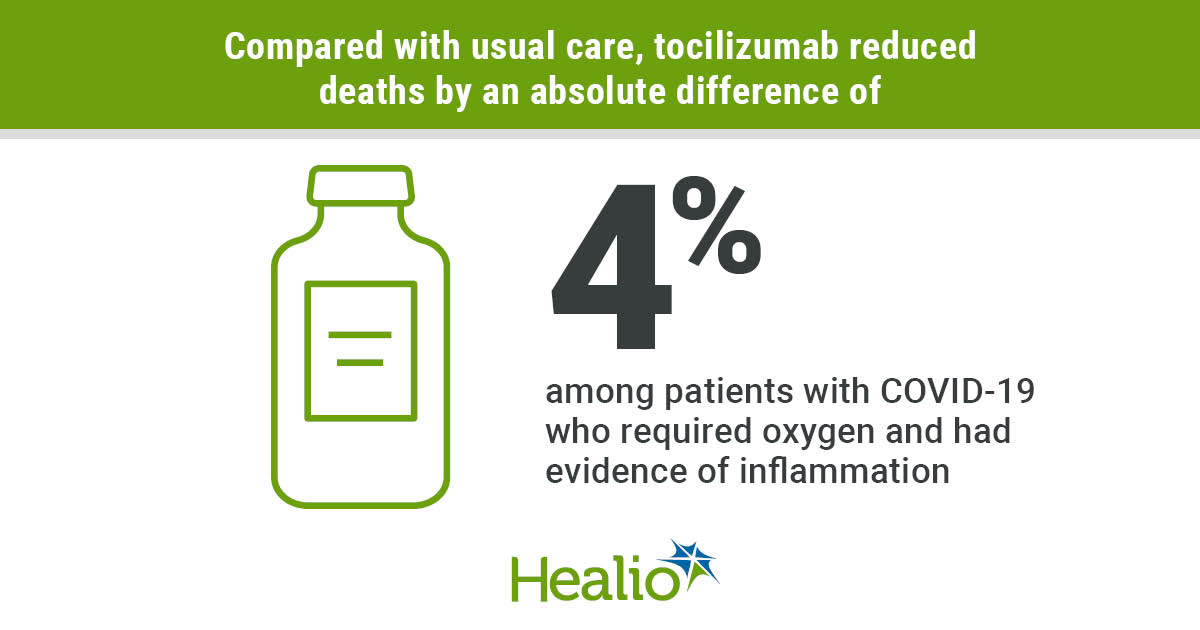
Unvaccinated Americans have died at 11 times the rate of those fully vaccinated since the delta variant became the dominant strain, indicate surveillance data gathered over the summer by the US Centers for Disease Control.
Vaccinated people were 10 times less likely to be admitted to hospital and five times less likely to be infected than unvaccinated people, found one study that tracked adults across 13 states and cities.1

 www.bmj.com
www.bmj.com
Unvaccinated Americans have died at 11 times the rate of those fully vaccinated since the delta variant became the dominant strain, indicate surveillance data gathered over the summer by the US Centers for Disease Control.
Vaccinated people were 10 times less likely to be admitted to hospital and five times less likely to be infected than unvaccinated people, found one study that tracked adults across 13 states and cities.1

Covid-19: Unvaccinated face 11 times risk of death from delta variant, CDC data show
Unvaccinated Americans have died at 11 times the rate of those fully vaccinated since the delta variant became the dominant strain, indicate surveillance data gathered over the summer by the US Centers for Disease Control. Vaccinated people were 10 times less likely to be admitted to hospital...